Page 10 - Curriculum Visions Dynamic Book
P. 10
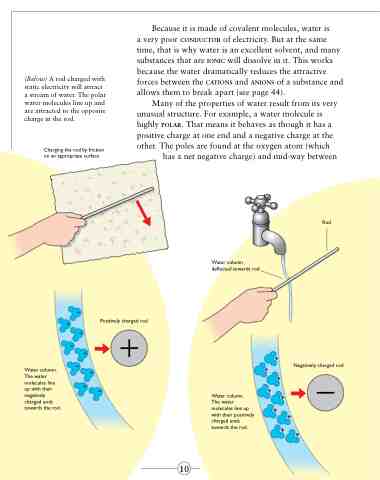
(Below) A rod charged with static electricity will attract a stream of water. The polar water molecules line up and are attracted to the opposite charge in the rod.
Charging the rod by friction on an appropriate surface
Because it is made of covalent molecules, water is
a very poor conductor of electricity. But at the same time, that is why water is an excellent solvent, and many substances that are ionic will dissolve in it. This works because the water dramatically reduces the attractive forces between the cations and anions of a substance and allows them to break apart (see page 44).
Many of the properties of water result from its very unusual structure. For example, a water molecule is highly polar. That means it behaves as though it has a positive charge at one end and a negative charge at the other. The poles are found at the oxygen atom (which
has a net negative charge) and mid-way between
Rod
Water column deflected towards rod
Positively charged rod
Water column. The water molecules line up with their negatively charged ends towards the rod.
Negatively charged rod
Water column.
The water molecules line up with their positively charged ends towards the rod.
10