Page 9 - Curriculum Visions Dynamic Book
P. 9
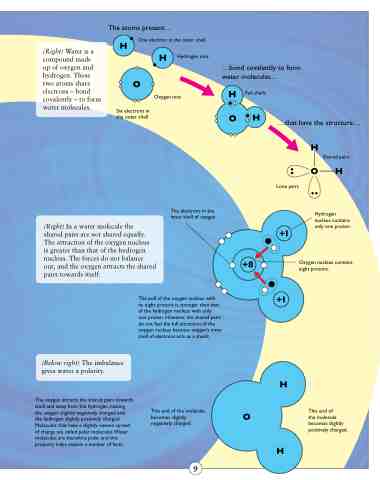
(Right) Water is a compound made
up of oxygen and hydrogen. These two atoms share electrons – bond covalently – to form water molecules.
The atoms present...
One electron in the outer shell
Hydrogen ions
Oxygen ions
...bond covalently to form water molecules...
Six electrons in the outer shell
...that have the structure... Shared pairs
Full shells
The electrons in the inner shell of oxygen
Hydrogen nucleus contains only one proton.
Oxygen nucleus contains eight protons.
Lone pairs
(Right) In a water molecule the
shared pairs are not shared equally. The attraction of the oxygen nucleus
is greater than that of the hydrogen nucleus. The forces do not balance out, and the oxygen attracts the shared pairs towards itself.
(Below right) The imbalance gives water a polarity.
The oxygen attracts the shared pairs towards itself and away from the hydrogen, making the oxygen slightly negatively charged and the hydrogen slightly positively charged. Molecules that have a slightly uneven spread of charge are called polar molecules.Water molecules are therefore polar, and this property helps explain a number of facts.
The pull of the oxygen nucleus with
its eight protons is stronger than that of the hydrogen nucleus with only
one proton. However, the shared pairs do not feel the full attraction of the oxygen nucleus because oxygen’s inner shell of electrons acts as a shield.
This end of the molecule becomes slightly negatively charged.
This end of
the molecule becomes slightly positively charged.
9