Page 12 - Curriculum Visions Dynamic Book
P. 12
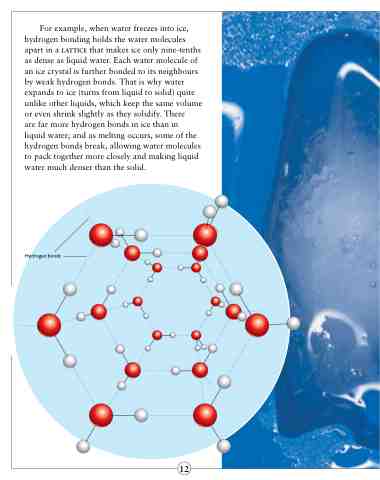
For example, when water freezes into ice, hydrogen bonding holds the water molecules apart in a lattice that makes ice only nine-tenths as dense as liquid water. Each water molecule of an ice crystal is further bonded to its neighbours by weak hydrogen bonds. That is why water expands to ice (turns from liquid to solid) quite unlike other liquids, which keep the same volume or even shrink slightly as they solidify. There
are far more hydrogen bonds in ice than in
liquid water; and as melting occurs, some of the hydrogen bonds break, allowing water molecules to pack together more closely and making liquid water much denser than the solid.
Hydrogen bonds
12