Page 34 - Curriculum Visions Dynamic Book. To close the book, close the tab.
P. 34
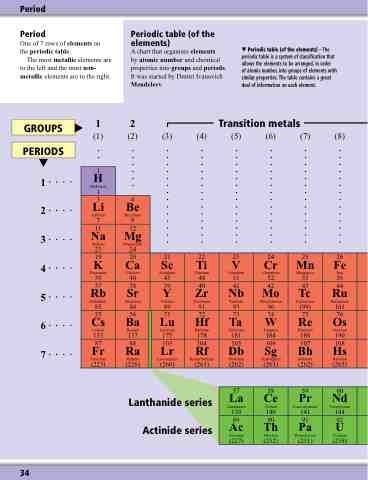
Period
Period
One of 7 rows of elements on the periodic table.
The most metallic elements are to the left and the most non- metallic elements are to the right.
34
19 20
Periodic table (of the elements)
A chart that organises elements
by atomic number and chemical properties into groups and periods. It was started by Dmitri Ivanovich Mendeleev.
Periodic table (of the elements) – The periodic table is a system of classification that allows the elements to be arranged, in order
of atomic number, into groups of elements with similar properties. The table contains a great deal of information on each element.
GROUPS
1... 2... 3... 4... 5... 6... 7...
1 2
(1) (2) (3)
Transition metals
PERIODS
1
21
(4) (5)
(6) (7) (8)
.H Hydrogen
1
. . . . . .
Li Be
Lithium Beryllium 79
11 12
Na Mg
Sodium Magnesium 23 24
22
23
24
25
26
K Ca
Potassium Calcium 39 40
37 38
Sc
Scandium 45
39
Ti
Titanium 48
40
V
Vanadium 51
Niobium 93
41
Cr
Chromium 52
Molybdenum 96
42
Mn
Manganese 55
43
Fe
Iron 56
Ruthenium 101
44
Rb Sr
Rubidium Strontium 85 88
55 56
Y
Yttrium 89
Lutetium 175
71
Zr
Zirconium 91
72
Nb
73
Mo
74
Tc
Technetium (99)
75
Ru
76
Cs Ba
Cesium Barium 133 137
87 88
Lu
103
Hf
Hafnium 178
104
Ta
Tantalum 181
105
W
Tungsten 184
106
Fr Ra
Francium Radium (223) (226)
Lr
Lawrencium (260)
Rf
Rutherfordium (261)
Db
Dubnium (262)
Sg
Seaborgium (263)
Re
Rhenium 186
107
Os
Osmium 190
108
Bh
Bohrium (262)
Hs
Hassium (265)
57
58
Lanthanide series Actinide series
La
Lanthanum 139
89
Ce
Cerium 140
90
59
60
Pr
Praseodymium 141
Nd
Neodymium 144
Ac
Actinium (227)
Th
Thorium (232)
91
Pa
Protactinium (231)
92
U
Uranium (238)
34
.............. .............. .............. .............. .............. .............. ......
..