Page 36 - Curriculum Visions Dynamic Book. To close the book, close the tab.
P. 36
Phosphorus (P)
Phosphorus (P)
Element 15 on the periodic table. A member of group 5 (the nitrogen group).
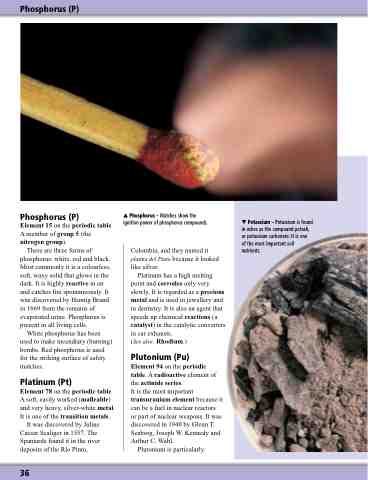
There are three forms of phosphorus: white, red and black. Most commonly it is a colourless, soft, waxy solid that glows in the dark. It is highly reactive in air and catches fire spontaneously. It was discovered by Hennig Brand in 1669 from the remains of evaporated urine. Phosphorus is present in all living cells.
White phosphorus has been used to make incendiary (burning) bombs. Red phosphorus is used for the striking surface of safety matches.
Platinum (Pt)
Element 78 on the periodic table. A soft, easily worked (malleable) and very heavy, silver-white metal. It is one of the transition metals.
It was discovered by Julius Caesar Scaliger in 1557. The Spaniards found it in the river deposits of the Río Pinto,
Phosphorus – Matches show the ignition power of phosphorus compounds.
Colombia, and they named it platina del Pinto because it looked like silver.
Platinum has a high melting point and corrodes only very slowly. It is regarded as a precious metal and is used in jewellery and in dentistry. It is also an agent that speeds up chemical reactions (a catalyst) in the catalytic converters in car exhausts.
(See also: Rhodium.)
Plutonium (Pu)
Element 94 on the periodic table. A radioactive element of the actinide series.
It is the most important transuranium element because it can be a fuel in nuclear reactors or part of nuclear weapons. It was discovered in 1940 by Glenn T. Seaborg, Joseph W. Kennedy and Arthur C. Wahl.
Plutonium is particularly
36
Potassium – Potassium is found in ashes as the compound potash, or potassium carbonate. It is one of the most important soil nutrients.