Page 49 - Curriculum Visions Dynamic Book
P. 49
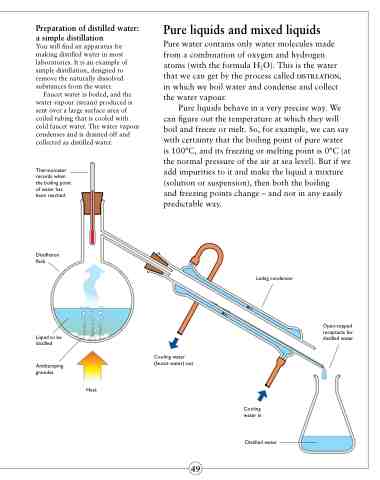
Preparation of distilled water:
a simple distillation
You will find an apparatus for making distilled water in most laboratories. It is an example of simple distillation, designed to remove the naturally dissolved substances from the water.
Faucet water is boiled, and the water vapour (steam) produced is sent over a large surface area of coiled tubing that is cooled with cold faucet water. The water vapour condenses and is drained off and collected as distilled water.
Pure liquids and mixed liquids
Pure water contains only water molecules made from a combination of oxygen and hydrogen atoms (with the formula H2O). This is the water that we can get by the process called distillation, in which we boil water and condense and collect the water vapour.
Pure liquids behave in a very precise way. We can figure out the temperature at which they will boil and freeze or melt. So, for example, we can say with certainty that the boiling point of pure water
is 100°C, and its freezing or melting point is 0°C (at the normal pressure of the air at sea level). But if we add impurities to it and make the liquid a mixture (solution or suspension), then both the boiling
and freezing points change – and not in any easily predictable way.
Thermometer records when the boiling point of water has been reached.
Distillation flask
Liquid to be distilled
Antibumping granules
Liebig condenser
Open-topped receptacle for distilled water
Heat
Cooling water (faucet water) out
Cooling water in
Distilled water
49