Page 47 - Curriculum Visions Dynamic Book
P. 47
Industrial separation of a salt
solution using electrolysis
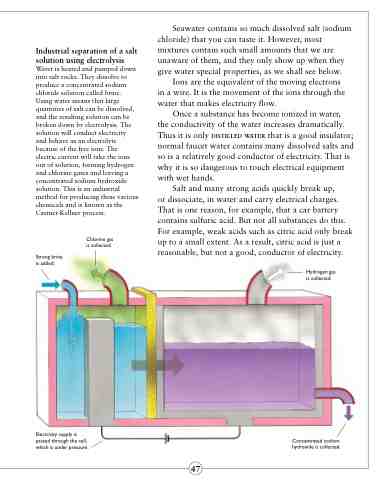
Water is heated and pumped down into salt rocks. They dissolve to produce a concentrated sodium chloride solution called brine. Using water means that large quantities of salt can be dissolved, and the resulting solution can be broken down by electrolysis. The solution will conduct electricity and behave as an electrolyte because of the free ions. The electric current will take the ions out of solution, forming hydrogen and chlorine gases and leaving a concentrated sodium hydroxide solution. This is an industrial method for producing these various chemicals and is known as the Castner-Kellner process.
Chlorine gas is collected.
Strong brine is added.
Seawater contains so much dissolved salt (sodium chloride) that you can taste it. However, most mixtures contain such small amounts that we are unaware of them, and they only show up when they give water special properties, as we shall see below.
Ions are the equivalent of the moving electrons in a wire. It is the movement of the ions through the water that makes electricity flow.
Once a substance has become ionized in water, the conductivity of the water increases dramatically. Thus it is only distilled water that is a good insulator; normal faucet water contains many dissolved salts and so is a relatively good conductor of electricity. That is why it is so dangerous to touch electrical equipment with wet hands.
Salt and many strong acids quickly break up,
or dissociate, in water and carry electrical charges. That is one reason, for example, that a car battery contains sulfuric acid. But not all substances do this. For example, weak acids such as citric acid only break up to a small extent. As a result, citric acid is just a reasonable, but not a good, conductor of electricity.
Hydrogen gas is collected.
Electricity supply is passed through the cell, which is under pressure.
Concentrated sodium hydroxide is collected.
47