Page 42 - Curriculum Visions Dynamic Book
P. 42
4: The universal solvent
A solution is produced when a solid, liquid, or gas becomes mixed in a liquid in such a way that it breaks up into molecules or ions. We then say that the substance has dissolved in the liquid. It is important to remember that substances dissolved (solutes) in the liquid (solvent) do not have themselves to be liquids. Blood and seawater, for example, both contain, among many other substances, oxygen gas (thus allowing animals to breathe).
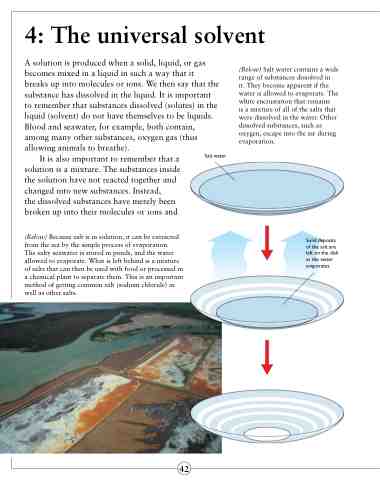
(Below) Salt water contains a wide range of substances dissolved in
it. They become apparent if the water is allowed to evaporate. The white encrustation that remains
is a mixture of all of the salts that were dissolved in the water. Other dissolved substances, such as oxygen, escape into the air during evaporation.
It is also important to remember that a solution is a mixture. The substances inside the solution have not reacted together and changed into new substances. Instead,
the dissolved substances have merely been broken up into their molecules or ions and
(Below) Because salt is in solution, it can be extracted from the sea by the simple process of evaporation.
The salty seawater is stored in ponds, and the water allowed to evaporate. What is left behind is a mixture of salts that can then be used with food or processed in a chemical plant to separate them. This is an important method of getting common salt (sodium chloride) as well as other salts.
Salt water
Solid deposits of the salt are left on the dish as the water evaporates.
42