Page 40 - Curriculum Visions Dynamic Book
P. 40
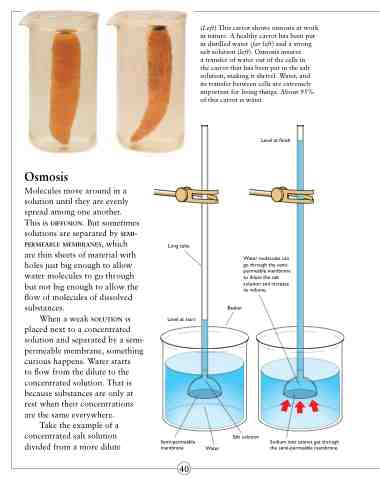
(Left) This carrot shows osmosis at work in nature. A healthy carrot has been put in distilled water (far left) and a strong salt solution (left). Osmosis insures
a transfer of water out of the cells in the carrot that has been put in the salt solution, making it shrivel. Water, and its transfer between cells are extremely important for living things. About 95% of this carrot is water.
Level at fnish
Osmosis
Molecules move around in a solution until they are evenly spread among one another. This is diffusion. But sometimes solutions are separated by semi- permeable membranes, which are thin sheets of material with holes just big enough to allow water molecules to go through but not big enough to allow the flow of molecules of dissolved substances.
When a weak solution is placed next to a concentrated solution and separated by a semi- permeable membrane, something curious happens. Water starts
to flow from the dilute to the concentrated solution. That is because substances are only at rest when their concentrations are the same everywhere.
Take the example of a concentrated salt solution divided from a more dilute
Long tube
Level at start
Water molecules can go through the semi- permeable membrane to dilute the salt solution and increase its volume.
Beaker
Semi-permeable membrane Water
Salt solution
Sodium ions cannot get through the semi-permeable membrane.
40