Page 44 - Curriculum Visions Dynamic Book
P. 44
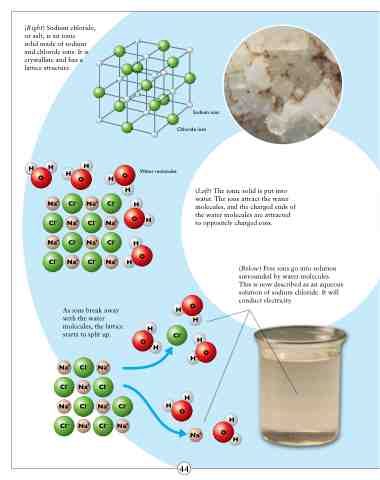
(Right) Sodium chloride, or salt, is an ionic
solid made of sodium and chloride ions. It is crystalline and has a lattice structure.
Water molecules
Sodium ions Chloride ions
(Left) The ionic solid is put into water. The ions attract the water molecules, and the charged ends of the water molecules are attracted to oppositely charged ions.
(Below) Free ions go into solution surrounded by water molecules. This is now described as an aqueous solution of sodium chloride. It will conduct electricity.
As ions break away with the water molecules, the lattice starts to split up.
44