Page 25 - Curriculum Visions Dynamic Book
P. 25
Making sodium chlorate
Sodium chlorate can be produced by the electrolysis of brine, a process similar to that taking place in the diaphragm cell. A variety of chlorates with differing amounts of oxygen can be made.
Sodium chlorate containing the least oxygen is used as a bleach (see page 14). Sodium chlorates with more oxygen, can be used in explosives, matches, fireworks, weedkillers, making oxygen (as shown below) and for throat lozenges.
EQUATION: Making sodium chlorate
decompose: to break down
a substance (for example by heat or with the aid of a catalyst) into simpler components. In such
a chemical reaction only one substance is involved.
explosive: a substance which, when a shock is applied to it, decomposes very rapidly, releasing a very large amount of heat and creating a large volume of gases as a shock wave.
Sodium chloride + water ➪ sodium hydroxide + chlorine + hydrogen 2NaCl(aq) + 2H2O(l) ➪ 2NaOH(aq) + Cl2(g) + H2(g)
Chlorine + sodium hydroxide ➪ sodium chlorate + water + sodium chloride 3Cl2(g) + 6NaOH(aq) ➪ NaClO3(aq) + 3H2O(l) + 5NaCl(aq)
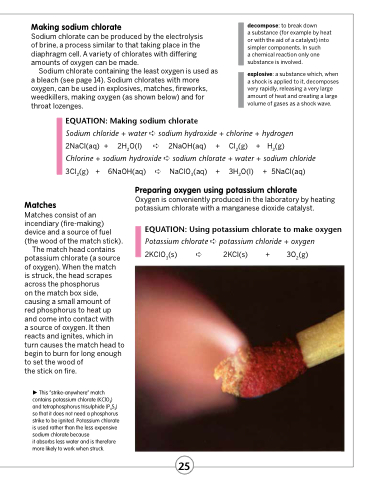
Matches
Matches consist of an incendiary (fire-making) device and a source of fuel (the wood of the match stick).
The match head contains potassium chlorate (a source of oxygen). When the match
is struck, the head scrapes across the phosphorus
on the match box side, causing a small amount of
red phosphorus to heat up and come into contact with
a source of oxygen. It then reacts and ignites, which in turn causes the match head to begin to burn for long enough to set the wood of
the stick on fire.
This “strike-anywhere” match contains potassium chlorate (KClO3) and tetraphosphorus trisulphide (P4S3) so that it does not need a phosphorus strike to be ignited. Potassium chlorate is used rather than the less expensive sodium chlorate because
it absorbs less water and is therefore more likely to work when struck.
Preparing oxygen using potassium chlorate
Oxygen is conveniently produced in the laboratory by heating potassium chlorate with a manganese dioxide catalyst.
EQUATION: Using potassium chlorate to make oxygen
Potassium chlorate ➪ potassium chloride + oxygen
2KClO3(s)
➪ 2KCl(s) + 3O2(g)
25
25