Page 23 - Curriculum Visions Dynamic Book
P. 23
The structure of polyvinyl chloride
Polyvinyl chloride is a hydrocarbon
chain, incorporating chlorine. The same structure but without the chlorine produces polythene, used to make plastic bags.
By substituting chlorine the properties
are changed so that the material is more durable and rigid.
hydrocarbon: a compound in which only hydrogen and carbon atoms are present. Most fuels are hydrocarbons, as is the simple plastic polyethene (known as polythene).
plastic (material): a carbon-based material consisting of long chains (polymers) of simple molecules. The word plastic is commonly restricted to synthetic polymers.
polymer: a compound that is made of long chains by combining molecules (called monomers) as repeating units. (“Poly” means many, “mer” means part).
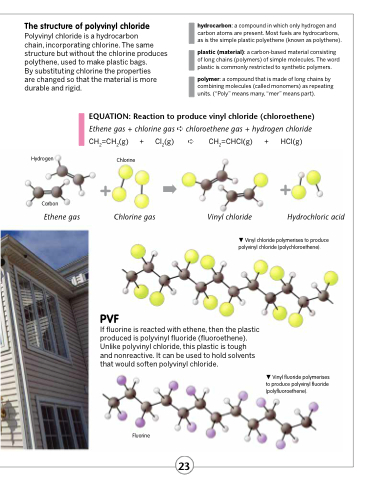
Hydrogen
Carbon
Ethene gas
EQUATION: Reaction to produce vinyl chloride (chloroethene)
Ethene gas + chlorine gas ➪ chloroethene gas + hydrogen chloride CH2=CH2(g) + Cl2(g) ➪ CH2=CHCl(g) + HCl(g)
Chlorine
+➡+
Chlorine gas Vinyl chloride Hydrochloric acid
PVF
If fluorine is reacted with ethene, then the plastic produced is polyvinyl fluoride (fluoroethene). Unlike polyvinyl chloride, this plastic is tough and nonreactive. It can be used to hold solvents that would soften polyvinyl chloride.
Fluorine
Vinyl fluoride polymerises to produce polyvinyl fluoride (polyfluoroethene).
Vinyl chloride polymerises to produce polyvinyl chloride (polychloroethene).
23
23