Page 26 - Curriculum Visions Dynamic Book
P. 26
Chlorine and poison gases
A poison gas is any gaseous chemical that can harm humans. In World War I the Germans made a poison gas attack at Ypres in Belgium. They put chlorine gas in cylinders under pressure and then released it when the wind was blowing towards the trenches containing Allied troops. At this time gas masks had not been invented.
Chlorine affects the cells of all the throat, mouth and lungs, causing damage to all the tissues and also causing fluids to be drawn out of the body and into the lungs. Death follows exposure for more than a couple of minutes.
Even more deadly is a combination of chlorine and cyanide. When this gas is
released and inhaled it prevents the red blood cells from carrying away waste carbon dioxide. This causes death within a very short time. Even in small doses, this terrible gas can affect the lungs and cause pneumonia. Mustard gas and phosgene – two of the most feared gases
of all – are also based on chlorine compounds.
Tear gas
Less harmful gases that irritate the throat and lungs and cause the tear glands to release fluids are called tear gases. Tear gases are used for crowd control in situations where there is risk
to life and property. Many tear gases contain chlorine. One of the most common is CN gas (chloroacetophenone). CS gas (chlorobenzylidenemalononitrile) acts faster and lasts longer than CN gas, causing involuntary closing of the eyes and a burning sensation. However, the effects disappear within a few minutes of being exposed to clean air.
Tear gases are effective at extremely low concentrations (refer back to the sensitivity of people to chlorine, page 10).
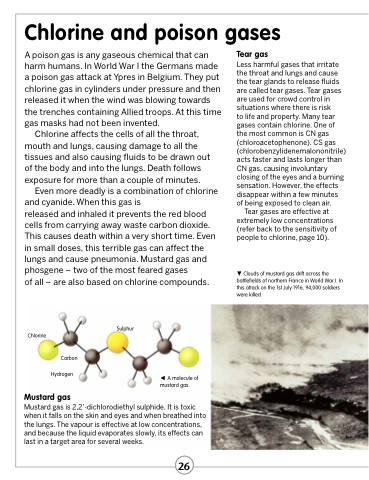
Clouds of mustard gas drift across the battlefields of northern France in World War I. In this attack on the 1st July 1916, 94,000 soldiers were killed.
Chlorine
Carbon Hydrogen
Mustard gas
A molecule of mustard gas.
Mustard gas is 2,2’-dichlorodiethyl sulphide. It is toxic when it falls on the skin and eyes and when breathed into the lungs. The vapour is effective at low concentrations, and because the liquid evaporates slowly, its effects can last in a target area for several weeks.
Sulphur
26
26