Page 20 - Curriculum Visions Dynamic Book
P. 20
Making chlorine: the diaphragm cell
The diaphragm cell is a piece of equipment that allows brine solution and sodium hydroxide to be used to manufacture a number of chemicals, including chlorine. It is an alternative to the mercury cathode cell process shown on the previous page.
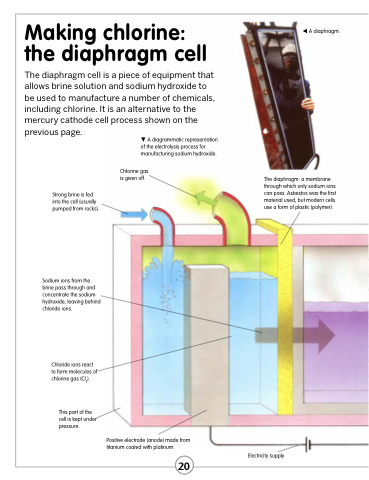
A diaphragm.
A diagrammatic representation of the electrolysis process for manufacturing sodium hydroxide.
Chlorine gas is given off.
The diaphragm: a membrane through which only sodium ions can pass. Asbestos was the first material used, but modern cells use a form of plastic (polymer).
Strong brine is fed into the cell (usually pumped from rocks).
Sodium ions from the brine pass through and concentrate the sodium hydroxide, leaving behind chloride ions.
Chloride ions react to form molecules of chlorine gas (Cl2).
This part of the cell is kept under pressure.
Positive electrode (anode) made from titanium coated with platinum.
Electricity supply
20
20