Page 19 - Curriculum Visions Dynamic Book
P. 19
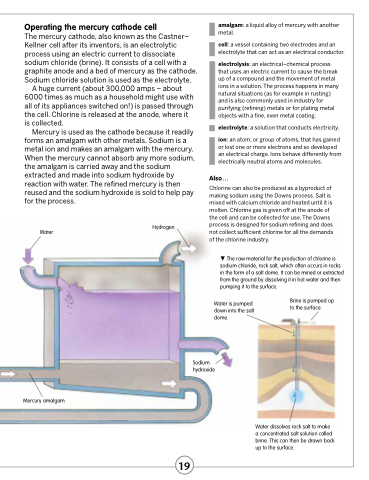
Operating the mercury cathode cell
The mercury cathode, also known as the Castner– Kellner cell after its inventors, is an electrolytic process using an electric current to dissociate sodium chloride (brine). It consists of a cell with a graphite anode and a bed of mercury as the cathode. Sodium chloride solution is used as the electrolyte.
A huge current (about 300,000 amps – about 6000 times as much as a household might use with all of its appliances switched on!) is passed through the cell. Chlorine is released at the anode, where it is collected.
amalgam: a liquid alloy of mercury with another metal.
cell: a vessel containing two electrodes and an electrolyte that can act as an electrical conductor.
electrolysis: an electrical–chemical process that uses an electric current to cause the break up of a compound and the movement of metal ions in a solution. The process happens in many natural situations (as for example in rusting) and is also commonly used in industry for purifying (refining) metals or for plating metal objects with a fine, even metal coating.
electrolyte: a solution that conducts electricity.
ion: an atom, or group of atoms, that has gained or lost one or more electrons and so developed an electrical charge. Ions behave differently from electrically neutral atoms and molecules.
Also...
Chlorine can also be produced as a byproduct of making sodium using the Downs process. Salt is mixed with calcium chloride and heated until it is molten. Chlorine gas is given off at the anode of the cell and can be collected for use. The Downs process is designed for sodium refining and does not collect sufficient chlorine for all the demands of the chlorine industry.
The raw material for the production of chlorine is sodium chloride, rock salt, which often occurs in rocks in the form of a salt dome. It can be mined or extracted from the ground by dissolving it in hot water and then pumping it to the surface.
Mercury is used as the cathode because it readily forms an amalgam with other metals. Sodium is a metal ion and makes an amalgam with the mercury. When the mercury cannot absorb any more sodium, the amalgam is carried away and the sodium extracted and made into sodium hydroxide by reaction with water. The refined mercury is then reused and the sodium hydroxide is sold to help pay for the process.
Water
Hydrogen
Mercury amalgam
Water is pumped down into the salt dome.
Sodium hydroxide
Brine is pumped up to the surface.
Water dissolves rock salt to make a concentrated salt solution called brine. This can then be drawn back up to the surface.
19
19