Page 26 - Curriculum Visions Dynamic Book
P. 26
Oxygen in iron and steelmaking
Iron and steelmaking are perhaps the largest example of redox reactions in the world. In the iron-making process (which takes place in a blast furnace) the iron oxide has to be reduced; in the steelmaking process, the remaining carbon has to be oxidised so it will leave the iron as a gas and make steel. The amount of oxygen required is huge. It amounts to about 80 kilograms per person in most industrial countries.
The majority of the oxygen is used in steelmaking, where three-quarters of a tonne of oxygen is needed to make every tonne of steel.
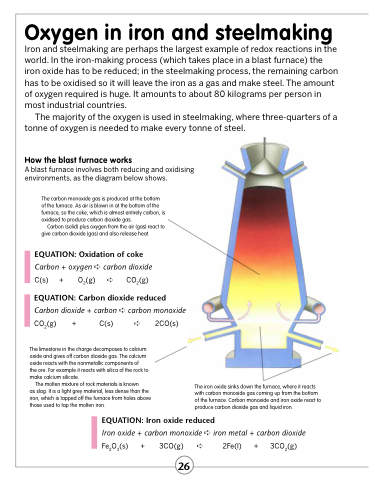
How the blast furnace works
A blast furnace involves both reducing and oxidising environments, as the diagram below shows.
The carbon monoxide gas is produced at the bottom of the furnace. As air is blown in at the bottom of the furnace, so the coke, which is almost entirely carbon, is oxidised to produce carbon dioxide gas.
Carbon (solid) plus oxygen from the air (gas) react to give carbon dioxide (gas) and also release heat.
EQUATION: Oxidation of coke
Carbon + oxygen ➪ carbon dioxide C(s) + O2(g) ➪ CO2(g)
EQUATION: Carbon dioxide reduced
Carbon dioxide + carbon ➪ carbon monoxide CO2(g) + C(s) ➪ 2CO(s)
The limestone in the charge decomposes to calcium oxide and gives off carbon dioxide gas. The calcium oxide reacts with the nonmetallic components of
the ore. For example it reacts with silica of the rock to make calcium silicate.
The molten mixture of rock materials is known
as slag. It is a light grey material, less dense than the iron, which is tapped off the furnace from holes above those used to tap the molten iron.
The iron oxide sinks down the furnace, where it reacts with carbon monoxide gas coming up from the bottom of the furnace. Carbon monoxide and iron oxide react to produce carbon dioxide gas and liquid iron.
EQUATION: Iron oxide reduced
Iron oxide + carbon monoxide ➪ iron metal + carbon dioxide Fe2O3(s) + 3CO(g) ➪ 2Fe(l) + 3CO2(g)
26
26