Page 24 - Curriculum Visions Dynamic Book
P. 24
Oxidation/reduction
Many of the reactions involving oxygen are reversible. That is, it is possible to add oxygen to a substance, a process called oxidation, and it is also possible to remove oxygen from a substance, a process called reduction.
Often the reversible process is called
a redox (reduction-oxidation) reaction. Here we demonstrate the principle of redox reactions and where to find them.
Also...Lead-acid batteries
A lead-acid battery consists of alternating lead and lead oxide plates soaked in an electrolyte of sulphuric acid. When the battery is connected to a load (for example a vehicle starter motor), oxidation occurs at the lead anode and complementary reduction occurs at
the lead oxide anode. Both changes release ions and so produce electricity. The chemical process is reversed when
a generator is attached to the battery.
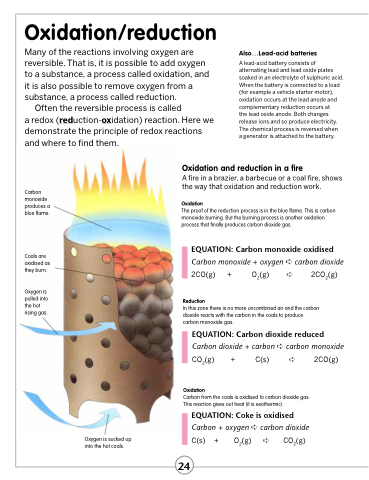
Carbon monoxide produces a blue flame.
Coals are oxidised as they burn.
Oxygen is pulled into the hot rising gas.
Oxidation and reduction in a fire
A fire in a brazier, a barbecue or a coal fire, shows the way that oxidation and reduction work.
Oxidation
The proof of the reduction process is in the blue flame. This is carbon monoxide burning. But the burning process is another oxidation process that finally produces carbon dioxide gas.
EQUATION: Carbon monoxide oxidised
Carbon monoxide + oxygen ➪ carbon dioxide 2CO(g) + O2(g) ➪ 2CO2(g)
Reduction
In this zone there is no more uncombined air and the carbon dioxide reacts with the carbon in the coals to produce carbon monoxide gas.
EQUATION: Carbon dioxide reduced
Carbon dioxide + carbon ➪ carbon monoxide CO2(g) + C(s) ➪ 2CO(g)
Oxidation
Carbon from the coals is oxidised to carbon dioxide gas. This reaction gives out heat (it is exothermic).
EQUATION: Coke is oxidised
Carbon + oxygen ➪ carbon dioxide C(s) + O2(g) ➪ CO2(g)
Oxygen is sucked up into the hot coals.
24
24