Page 27 - Curriculum Visions Dynamic Book
P. 27
Steelmaking
The first steelmaking plant was known as the Bessemer Converter. A large metal container
was lined with dolomite (calcium magnesium carbonate). When the container is heated the dolomite decomposed and combined with the impurities in the iron to make a slag that was drawn off. To help the process of oxidation further, air was blown through the container. The carbon in the iron oxidised to give carbon monoxide, a gas that then bubbled out of the steel.
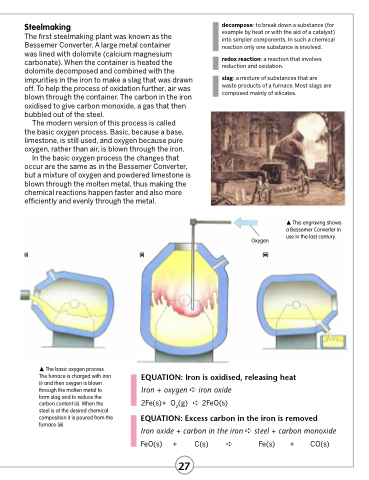
The modern version of this process is called the basic oxygen process. Basic, because a base, limestone, is still used, and oxygen because pure oxygen, rather than air, is blown through the iron.
In the basic oxygen process the changes that occur are the same as in the Bessemer Converter, but a mixture of oxygen and powdered limestone is blown through the molten metal, thus making the chemical reactions happen faster and also more efficiently and evenly through the metal.
decompose: to break down a substance (for example by heat or with the aid of a catalyst) into simpler components. In such a chemical reaction only one substance is involved.
redox reaction: a reaction that involves reduction and oxidation.
slag: a mixture of substances that are waste products of a furnace. Most slags are composed mainly of silicates.
(i)
(ii)
The basic oxygen process. The furnace is charged with iron (i) and then oxygen is blown through the molten metal to form slag and to reduce the carbon content (ii). When the steel is at the desired chemical composition it is poured from the furnace (iii).
EQUATION: Iron is oxidised, releasing heat
Iron + oxygen ➪ iron oxide 2Fe(s)+ O2(g) ➪ 2FeO(s)
EQUATION: Excess carbon in the iron is removed
Iron oxide + carbon in the iron ➪ steel + carbon monoxide
FeO(s) +
Oxygen
(iii)
This engraving shows a Bessemer Converter in use in the last century.
C(s) ➪ Fe(s) + CO(s)
27
27