Page 8 - Curriculum Visions Dynamic Book
P. 8
Crystals of carbon
Three minerals – graphite, diamond and the more recently discovered buckminsterfullerene (known as “buckyballs”) – are made solely
of carbon atoms. Of these, graphite is the
most common. It occurs in rocks, and
it is also formed as small crystals when hydrocarbons burn in the absence of air
(e.g. coke, charcoal).
Diamond is far more rare than graphite. Diamonds were formed under immense temperatures and pressures, such as found in pipes leading to ancient volcanoes. The most famous diamond mine, at Kimberley, South Africa, follows an old volcanic pipe for more than two kilometres vertically into the Earth.
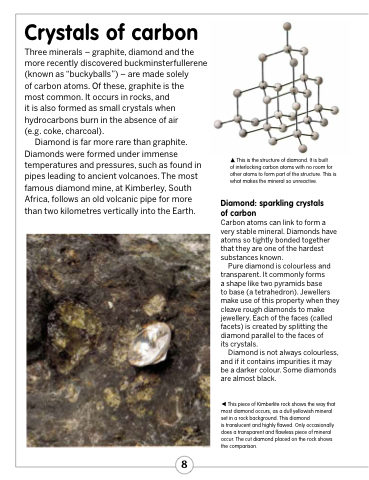
This is the structure of diamond. It is built
of interlocking carbon atoms with no room for other atoms to form part of the structure. This is what makes the mineral so unreactive.
Diamond: sparkling crystals
of carbon
Carbon atoms can link to form a very stable mineral. Diamonds have atoms so tightly bonded together that they are one of the hardest substances known.
Pure diamond is colourless and transparent. It commonly forms
a shape like two pyramids base
to base (a tetrahedron). Jewellers make use of this property when they cleave rough diamonds to make jewellery. Each of the faces (called facets) is created by splitting the diamond parallel to the faces of
its crystals.
Diamond is not always colourless, and if it contains impurities it may be a darker colour. Some diamonds are almost black.
This piece of Kimberlite rock shows the way that most diamond occurs, as a dull yellowish mineral set in a rock background. This diamond
is translucent and highly flawed. Only occasionally does a transparent and flawless piece of mineral occur. The cut diamond placed on the rock shows the comparison.
8