Page 6 - Curriculum Visions Dynamic Book
P. 6
What do carbon compounds have in common?
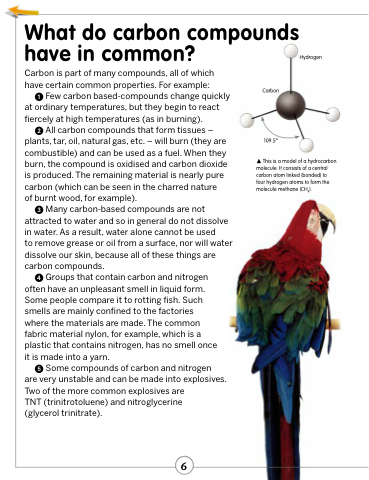
Hydrogen
Carbon is part of many compounds, all of which have certain common properties. For example:
Few carbon based-compounds change quickly at ordinary temperatures, but they begin to react fiercely at high temperatures (as in burning).
All carbon compounds that form tissues – plants, tar, oil, natural gas, etc. – will burn (they are combustible) and can be used as a fuel. When they burn, the compound is oxidised and carbon dioxide is produced. The remaining material is nearly pure carbon (which can be seen in the charred nature
of burnt wood, for example).
Many carbon-based compounds are not attracted to water and so in general do not dissolve in water. As a result, water alone cannot be used
to remove grease or oil from a surface, nor will water dissolve our skin, because all of these things are carbon compounds.
Groups that contain carbon and nitrogen often have an unpleasant smell in liquid form. Some people compare it to rotting fish. Such smells are mainly confined to the factories where the materials are made. The common fabric material nylon, for example, which is a plastic that contains nitrogen, has no smell once it is made into a yarn.
Some compounds of carbon and nitrogen
are very unstable and can be made into explosives. Two of the more common explosives are
TNT (trinitrotoluene) and nitroglycerine
(glycerol trinitrate).
6 6
Carbon
109.5°
This is a model of a hydrocarbon molecule. It consists of a central carbon atom linked (bonded) to four hydrogen atoms to form the molecule methane (CH4).