Page 17 - Curriculum Visions Dynamic Book
P. 17
solution: a mixture of a liquid and at least one other substance (e.g. salt water). Mixtures can be separated out by physical means, for example by evaporation and cooling.
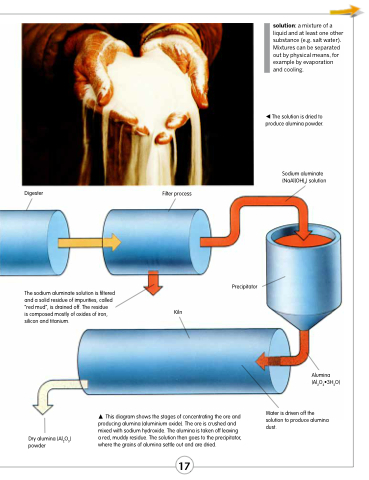
The solution is dried to produce alumina powder.
Sodium aluminate (NaAl(OH)4) solution
Digester
Filter process
The sodium aluminate solution is filtered and a solid residue of impurities, called “red mud”, is drained off. The residue
is composed mostly of oxides of iron, silicon and titanium.
Precipitator
This diagram shows the stages of concentrating the ore and producing alumina (aluminium oxide). The ore is crushed and mixed with sodium hydroxide. The alumina is taken off leaving a red, muddy residue. The solution then goes to the precipitator, where the grains of alumina settle out and are dried.
Alumina (Al2O3•3H2O)
Water is driven off the solution to produce alumina dust.
Kiln
Dry alumina (Al2O3) powder
17
17