Page 19 - Curriculum Visions Dynamic Book
P. 19
EQUATION: Overall equations for the reduction of alumina to aluminium
Alumina ➪ aluminium + oxygen 2Al O (s) ➪ 4Al(s) + 3O (g)
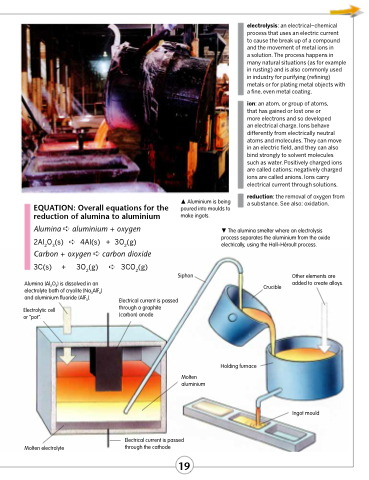
Aluminium is being poured into moulds to make ingots.
electrolysis: an electrical–chemical process that uses an electric current to cause the break up of a compound and the movement of metal ions in
a solution. The process happens in many natural situations (as for example in rusting) and is also commonly used in industry for purifying (refining) metals or for plating metal objects with a fine, even metal coating.
ion: an atom, or group of atoms,
that has gained or lost one or
more electrons and so developed
an electrical charge. Ions behave differently from electrically neutral atoms and molecules. They can move in an electric field, and they can also bind strongly to solvent molecules such as water. Positively charged ions are called cations; negatively charged ions are called anions. Ions carry electrical current through solutions.
reduction: the removal of oxygen from a substance. See also: oxidation.
The alumina smelter where an electrolysis process separates the aluminium from the oxide electrically, using the Hall–Héroult process.
2 3 2
Carbon + oxygen ➪ carbon dioxide 3C(s) + 3O2(g) ➪ 3CO2(g)
Siphon
Alumina (Al2O3) is dissolved in an electrolyte bath of cryolite (Na3AlF6) and aluminium fluoride (AlF3).
Electrolytic cell or “pot”.
Crucible
Other elements are added to create alloys.
Molten electrolyte
Electrical current is passed through a graphite (carbon) anode
Molten aluminium
Electrical current is passed through the cathode
Holding furnace
Ingot mould
19
19