Page 43 - Curriculum Visions Dynamic Book
P. 43
The gas that tells of success
The action of a carbonate antacid is to produce a gas. When this builds up in the digestive system, it is released quite
uncontrollably as a “burp.”
However, the gas that occurs in some fizzing
antacid tablets is formed in the glass. This is because the manufacturers put citric acid powder in with the calcium carbonate.
acid: compounds containing hydrogen that can attack and dissolve many substances.
neutralization: the reaction of acids
and bases to produce a salt and water.
The reaction causes hydrogen from the acid and hydroxide from the base to
be changed to water. For example, hydrochloric acid reacts with sodium hydroxide to form common salt and water. The term is more generally used for any reaction in which the pH changes toward 7.0, which is the pH of a neutral solution.
When the tablet is put in water, the acid and carbonate react to release
carbon dioxide and give an attractive impression of action
even before the antacid is digested.
Carbon dioxide gas in belch
Why too much acid is produced
The digestive system is triggered into releasing acids to match the volume of food we eat. So if we eat a lot, a great deal of acid is released from the linings of the digestive system.
Acids work on the surfaces of food, so the better it is chewed, the faster the acids can get to work. If food is eaten in large lumps (i.e., it
is bolted down), the acid will not be able to get to it quickly. As a result the amount of acid produced can be more than is needed for digestion. The excess acid then starts to attack the linings of the digestive system, causing the pains that are known as indigestion and heartburn. Bacteria working in this very acid environment can even cause ulcers.
Acid released from stomach lining
Calcium carbonate reacts with acids
EQUATION: Neutralizing stomach acid with a carbonate
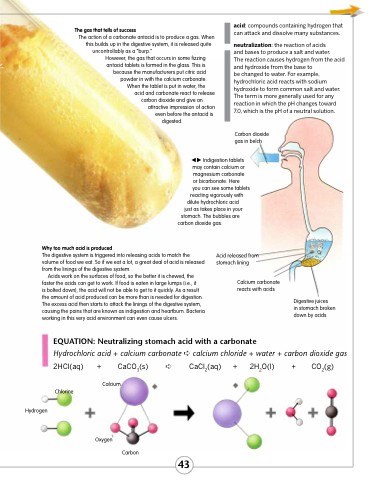
Indigestion tablets may contain calcium or magnesium carbonate
or bicarbonate. Here
you can see some tablets
reacting vigorously with dilute hydrochloric acid
just as takes place in your stomach. The bubbles are
carbon dioxide gas.
Hydrochloric acid + calcium carbonate ➪ calcium chloride + water + carbon dioxide gas
2HCl(aq)
Chlorine Hydrogen
+ CaCO3(s) ➪ CaCl2(aq) + 2H2O(l) + CO2(g) Calcium
Oxygen
Carbon
Digestive juices
in stomach broken down by acids
43 43