Page 42 - Curriculum Visions Dynamic Book
P. 42
Antacids
The body makes strong acids to help to digest food. Most of these acids are produced in the lining of the stomach.
The acids are vital and usually the food neutralizes the acids exactly, so that no discomfort is produced. But under some circumstances, especially if we eat a large amount of food and do not chew it properly, the digestive system does not balance.
Neutralizing
One way to counteract the occasional problem of indigestion is to use a substance, such as calcium carbonate, that reacts with an acid. “Antacids” are normally taken in powder form, as a tablet, or sometimes as a suspension in water. Calcium bicarbonate is also used because it is soluble and can be mixed more easily with water.
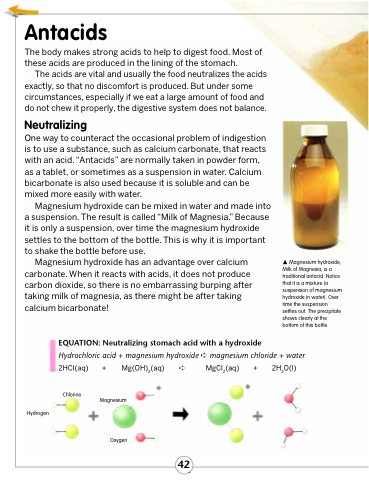
Magnesium hydroxide can be mixed in water and made into a suspension. The result is called “Milk of Magnesia.” Because it is only a suspension, over time the magnesium hydroxide settles to the bottom of the bottle. This is why it is important to shake the bottle before use.
Magnesium hydroxide has an advantage over calcium carbonate. When it reacts with acids, it does not produce carbon dioxide, so there is no embarrassing burping after taking milk of magnesia, as there might be after taking calcium bicarbonate!
EQUATION: Neutralizing stomach acid with a hydroxide
Magnesium hydroxide, Milk of Magnesia, is a traditional antacid. Notice that it is a mixture (a suspension of magnesium hydroxide in water). Over time the suspension settles out. The precipitate shows clearly at the bottom of this bottle.
2HCl(aq)
Chlorine Hydrogen
+ Mg(OH)2(aq) ➪ MgCl2(aq) + 2H2O(l) Magnesium
42 42
Hydrochloric acid + magnesium hydroxide ➪ magnesium chloride + water
Oxygen