Page 40 - Curriculum Visions Dynamic Book
P. 40
Softening water
Hard water – water containing a
large amount of dissolved calcium and magnesium salts – can be difficult to use
for washing. It makes the wash water develop a soapy scum that is hard to remove. This means that it is very difficult to get cutlery and crockery “squeaky clean,” making everything less pleasant to look at.
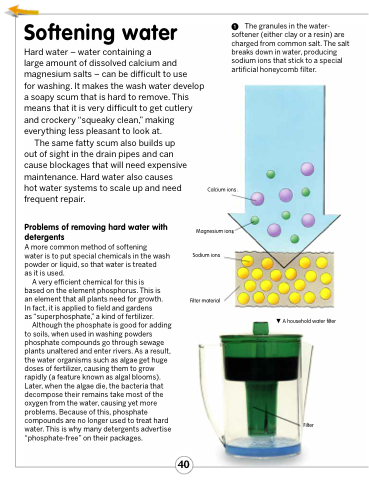
The granules in the water- softener (either clay or a resin) are charged from common salt. The salt breaks down in water, producing sodium ions that stick to a special artificial honeycomb filter.
The same fatty scum also builds up out of sight in the drain pipes and can cause blockages that will need expensive maintenance. Hard water also causes hot water systems to scale up and need frequent repair.
Problems of removing hard water with detergents
A more common method of softening
water is to put special chemicals in the wash powder or liquid, so that water is treated
as it is used.
A very efficient chemical for this is
based on the element phosphorus. This is an element that all plants need for growth. In fact, it is applied to field and gardens
as “superphosphate,” a kind of fertilizer.
Although the phosphate is good for adding to soils, when used in washing powders phosphate compounds go through sewage plants unaltered and enter rivers. As a result, the water organisms such as algae get huge doses of fertilizer, causing them to grow rapidly (a feature known as algal blooms). Later, when the algae die, the bacteria that decompose their remains take most of the oxygen from the water, causing yet more problems. Because of this, phosphate compounds are no longer used to treat hard water. This is why many detergents advertise “phosphate-free” on their packages.
Calcium ions
Magnesium ions Sodium ions
Filter material
A household water filter
Filter
40 40