Page 28 - Curriculum Visions Dynamic Book
P. 28
Manufacturing sodium
carbonate
Sodium carbonate (a chemical
needed for soap and glass-making) was originally made by soaking plants in water. However, this used a huge amount of plant material and produced very little chemical.
The first scientifically designed chemical process to make sodium carbonate was invented by Nicolas Leblanc in 1789. He poured sulphuric acid on to common salt and then treated the result with limestone and charcoal.
In 1861, a less complicated and far more efficient process was invented
by the Belgian scientist, Ernest Solvay. The Solvay process begins with a
strong brine solution, through which ammonia and carbon dioxide are then bubbled. The three substances then combine to produce sodium bicarbonate (baking soda). When this is heated,
it forms sodium carbonate.
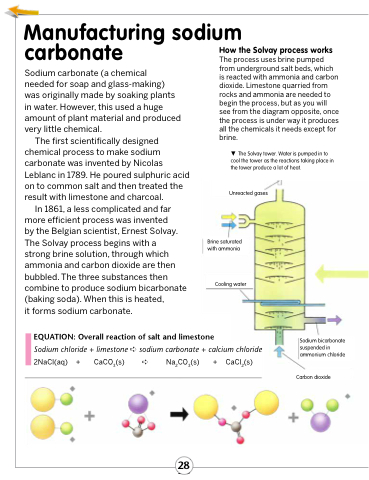
How the Solvay process works
The process uses brine pumped from underground salt beds, which is reacted with ammonia and carbon dioxide. Limestone quarried from rocks and ammonia are needed to begin the process, but as you will see from the diagram opposite, once the process is under way it produces all the chemicals it needs except for brine.
The Solvay tower. Water is pumped in to cool the tower as the reactions taking place in the tower produce a lot of heat.
Unreacted gases
Brine saturated with ammonia
Cooling water
Sodium chloride + limestone ➪ sodium carbonate + calcium chloride 2NaCl(aq) + CaCO3(s) ➪ Na2CO3(s) + CaCl2(s)
EQUATION: Overall reaction of salt and limestone
Sodium bicarbonate suspended in ammonium chloride
Carbon dioxide
28 28