Page 28 - Curriculum Visions Dynamic Book
P. 28
Carbonic acid
Carbonic acid, H2CO3, is a weak acid formed by the reaction of water and carbon dioxide gas.
Carbonic acid occurs in places where water and carbon dioxide are found naturally: in the atmosphere, where carbon dioxide gas dissolves in rainwater droplets; in the body, where it is formed in the stomach (along with hydrochloric acid), lungs and blood; and in the soil where soil organisms respire and cause a build- up of carbon dioxide gas.
Carbonic acid is responsible for the weathering of many limestone landscapes; it is partly responsible for the formation of caves and for the weathering of limestone buildings.
Carbonic acid occurs in the body as carbon dioxide reacts with water in the blood and elsewhere. As a result, the body has to have mechanisms (such as the kidneys) to regulate the amount, or an excessive build-up of carbonic acid will occur, giving rise
to a disorder called acidosis.
In a healthy person, body fluids are heavily buffered with both bicarbonates and carbonic acid. The purpose of the buffers is to allow the blood to maintain itself as a neutral solution with a pH close to 7.
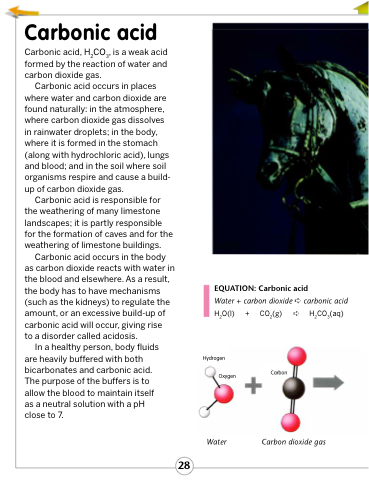
EQUATION: Carbonic acid
Water + carbon dioxide ➪ carbonic acid
H2O(l)
Hydrogen Oxygen
+
CO2(g) ➪ H2CO3(aq)
Carbon
Water
Carbon dioxide gas
28