Page 6 - Curriculum Visions Dynamic Book
P. 6
Gallium (Ga)
Element 31. Gallium is a rare silvery- white metal in the boron group, which is group 3 in the Periodic Table.The solid metal is brittle and breaks with a conchoidal fracture like glass.The metal expands on solidifying and has a melting point just above room temperature. Some gallium compounds emit light when an electric current passes through them.
Discovery
Gallium was discovered by Paul-Émile Lecoq de Boisbaudran in 1875 as an impurity in zinc blende (sulphide).
Technology
Its low melting point and high boiling point make it possible to use it in high- temperature thermometers. Gallium arsenide goes into making LEDs (light- emitting diodes). It is also used for doping semi-conductors. It makes a brilliant mirror when painted on glass. Ninety tonnes of gallium in large tanks (three years of
world production) are used to detect solar neutrinos.
Geology
Gallium is not found as a native element. It is normally recovered as a by-product of other ores.
Biology
It has no biological role.
Key facts...
Name: gallium
Symbol: Ga
Atomic number: 31
Atomic weight: 69.72
Position in Periodic Table: group 3 (13) (boron
group); period 4
State at room temperature: solid
Colour: silvery-white
Density of solid: 5.90 g/cc
Melting point: 29.76°C
Boiling point: 2,403°C
Origin of name: from the Latin word Gallia,
meaning France
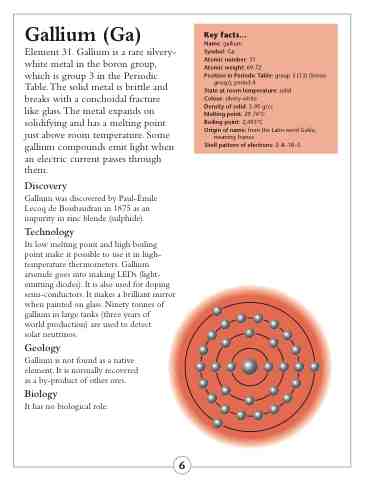
Shell pattern of electrons: 2–8–18–3
6