Page 4 - Curriculum Visions Dynamic Book
P. 4
Fm see Fermium Francium (Fr)
Element 87. Francium is the heaviest and most reactive alkali metal in group 1 in the Periodic Table.
It is a radioactive metal that
only occurs in the most minute quantities. It is the most unstable of the first 101 elements, with a half- life of 22 minutes. Its properties are very similar to those of cesium.
Discovery
Marguerite Perey of the Curie Institute in Paris discovered francium in 1939. However, it has never been isolated as the pure element. Because it is so radioactive, it quickly decays to other elements.The longest lived isotope, francium-223, has
a half-life of 22 minutes. It is the only isotope of francium that occurs in nature.
Technology
It has no uses. It can be produced artificially by bombarding thorium with protons.
Geology
Natural francium is present in only the minutest of quantities in nature, perhaps less than 30g in the entire Earth’s crust at any one time. It is produced by the disintegration of actinium. It is found in uranium minerals.
Biology
As a radioactive element it is theoretically harmful but it is produced only in tiny quantities.
Key facts...
Name: francium
Symbol: Fr
Atomic number: 87
Atomic weight: 223
Position in Periodic Table: group 1 (1) (alkali
metal); period 7
State at room temperature: solid
Colour: metallic
Density: n/a
Melting point: 27°C
Boiling point: 677°C
Origin of name: named after France
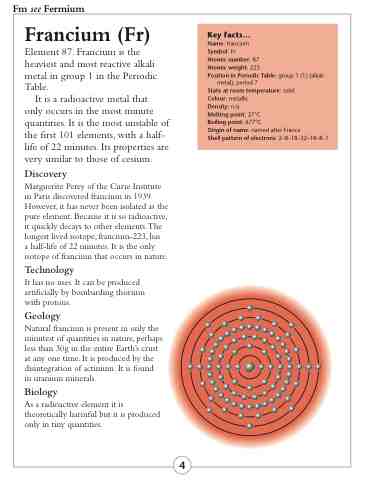
Shell pattern of electrons: 2–8–18–32–18–8–1
4