Page 8 - Curriculum Visions Dynamic Book
P. 8
Gold (Au)
Element 79. Gold is a dense, heavy, shiny, yellow precious metal and one of the transition metals in the Periodic Table.
Gold is soft and easily shaped
– even making the thin sheets called gold leaf. One gram (ounce) of gold can be beaten to a sheet of gold that covers 15 sq m (300 sq ft). It does not tarnish or corrode.
Its traditional role in coins and low reactivity have made it the one material that can be used worldwide instead of money.
Gold is a good conductor of heat and electricity, and it is plated over switch contacts and other places where it is important that no corrosion occurs. Gold has also long been used for tooth fillings, partly because it doesn’t corrode, and partly for its decorative effect.
Key facts...
Name: gold
Symbol: Au
Atomic number: 79
Atomic weight: 196.96
Position in Periodic Table: transition metal, group
(11) (copper group; coinage metal); period 6 State at room temperature: solid
Colour: yellow-metallic
Density of solid: 19.3
Melting point: 1,063°C
Boiling point: 2,966°C
Origin of name: from the Anglo-Saxon word gold;
the symbol Au is from the Latin word aurum,
meaning gold.
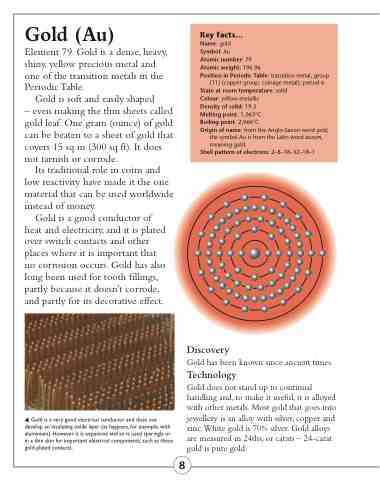
Shell pattern of electrons: 2–8–18–32–18–1
Gold is a very good electrical conductor and does not develop an insulating oxide layer (as happens, for example, with aluminium). However it is expensive and so is used sparingly or in a thin skin for important electrical components, such as these gold-plated contacts.
Discovery
Gold has been known since ancient times.
Technology
Gold does not stand up to continual handling and, to make it useful, it is alloyed with other metals. Most gold that goes into jewellery is an alloy with silver, copper and zinc.White gold is 70% silver. Gold alloys are measured in 24ths, or carats – 24-carat gold is pure gold.
8