Page 41 - Curriculum Visions Dynamic Book
P. 41
Nitrogen (N)
Element 7.An almost inert (unreactive) gas at ordinary temperature and pressure. It is a colourless, odourless and tasteless non-metallic element in group 5 (the nitrogen group) in the Periodic Table. Nitrogen is the sixth most common element in the universe. It is the most common element in the atmosphere, making up 78% of the air and it is also found in all living matter.
Nitrogen is made by liquefying air.
When nitrogen is heated, it
combines with magnesium, lithium,
and calcium. Under pressure it
combines with hydrogen to form ammonia. This is called the Haber process.When mixed with oxygen and in the presence
Key facts...
Name: nitrogen
Symbol: N
Atomic number: 7
Atomic weight: 14.01
Position in Periodic Table: group 5 (15)
(nitrogen group); period 2
State at room temperature: gas
Colour: colourless
Density of gas at 20°C: 1.17 g/l
Melting point: –209.86°C
Boiling point: –196°C
Origin of name: the French chemist Antoine
Lavoisier named it azote because no living thing could survive in it (from the Greek word meaning life). This term is still used in French. The name nitrogen was coined in 1790 after a common mineral then known as nitre (potassium nitrate) from niter and -gen, meaning nitre-forming.
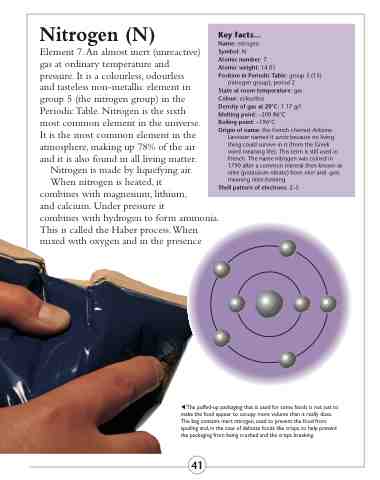
Shell pattern of electrons: 2–5
The puffed-up packaging that is used for some foods is not just to make the food appear to occupy more volume than it really does. The bag contains inert nitrogen, used to prevent the food from spoiling and, in the case of delicate foods like crisps, to help prevent the packaging from being crushed and the crisps breaking.
41