Page 26 - Curriculum Visions Dynamic Book
P. 26
Lithium (Li)
Element 3.A member of the alkali metals, group 1 in the Periodic Table.
Lithium is a soft, silvery-white, shiny metal that has only half the density of water. It is very reactive and rapidly tarnishes in air. Lithium metal has the highest specific heat of any solid element. Lithium salts colour flames bright red.
Discovery
Lithium was discovered in Stockholm, Sweden in 1817 by Johan August Arfwedson.
Technology
Lithium is alloyed with aluminium, lead, and other soft metals to make them harder and so more useful in, for example, aircraft manufacture. Lithium also goes into some batteries as well
as high-strength glass. Lithium is added
to oils to make a high-temperature lubricant. Lithium hydroxide can
absorb carbon dioxide in space vehicles.
Geology
Because it is highly reactive, lithium does not occur as a native element. It is found in most igneous rocks as a minor component.
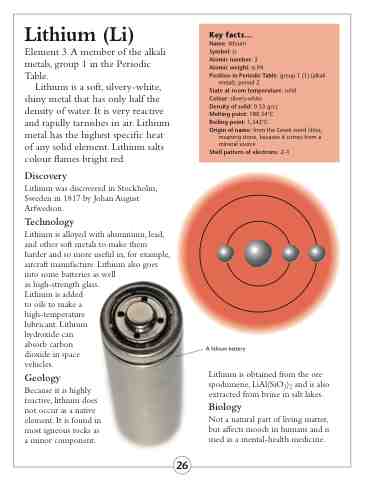
A lithium battery
Lithium is obtained from the ore spodumene, LiAl(SiO3)2 and is also extracted from brine in salt lakes.
Biology
Not a natural part of living matter, but affects moods in humans and is used as a mental-health medicine.
Key facts...
Name: lithium
Symbol: Li
Atomic number: 3
Atomic weight: 6.94
Position in Periodic Table: group 1 (1) (alkali
metal); period 2
State at room temperature: solid
Colour: silvery-white
Density of solid: 0.53 g/cc
Melting point: 180.54°C
Boiling point: 1,342°C
Origin of name: from the Greek word lithos,
meaning stone, because it comes from a
mineral source
Shell pattern of electrons: 2–1
26