Page 27 - Curriculum Visions Dynamic Book
P. 27
Lr see Lawrencium Lutetium (Lu)
Element 71. It is a rare-earth element and belongs to the transition metals in the Periodic Table. It is a silvery-white metal that reacts slowly and so is quite stable in air.
Discovery
Lutetium was discovered separately in France in 1907 by Georges Urbain and
in Austria by Carl Auer von Welsbach. Georges Urbain named the element lutetium, which was based on the word Lutetia, the Latin name for Paris. Lutetium was spelled ‘lutecium’ until 1949.
Technology
A natural radioactive isotope of lutetium (with a half-life of 30 billion years) is used to date meteorites in comparison with the age of the Earth. Lutetium can help break up (crack) crude oil and can make some alloys. However, it is very expensive to prepare.
Geology
Lutetium is never found as a native metal, but is found in the minerals monazite
(a phosphate mineral) and bastnasite
(a carbonate mineral), as well as in
all minerals that contain yttrium.
Biology
Lutetium is not found in living things, but is not regarded as especially harmful.
Key facts...
Name: lutetium
Symbol: Lu
Atomic number: 71
Atomic weight: 174.96
Position in Periodic Table: transition metal, group
(3) (scandium group); period 6
State at room temperature: solid
Colour: silvery-white
Density of solid: 9.84 g/cc
Melting point: 1,652°C
Boiling point: 3,315°C
Origin of the name: from the Latin Lutetia,
meaning Paris
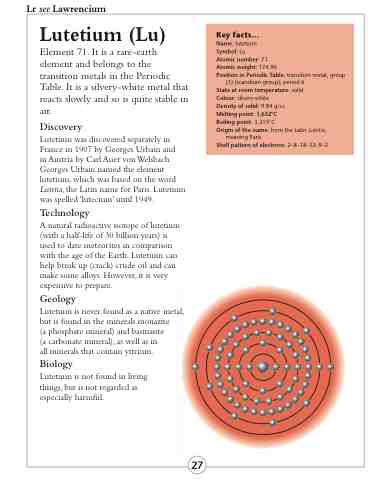
Shell pattern of electrons: 2–8–18–32–9–2
27