Page 55 - Curriculum Visions Dynamic Book
P. 55
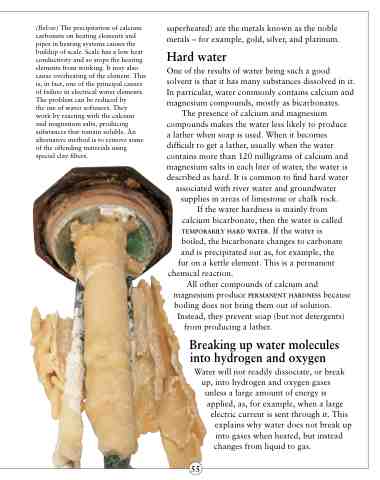
(Below) The precipitation of calcium carbonate on heating elements and pipes in heating systems causes the buildup of scale. Scale has a low heat conductivity and so stops the heating elements from working. It may also cause overheating of the element. This is, in fact, one of the principal causes of failure in electrical water elements. The problem can be reduced by
the use of water softeners. They work by reacting with the calcium and magnesium salts, producing substances that remain soluble. An alternative method is to remove some of the offending materials using special clay filters.
superheated) are the metals known as the noble metals – for example, gold, silver, and platinum.
Hard water
One of the results of water being such a good solvent is that it has many substances dissolved in it. In particular, water commonly contains calcium and magnesium compounds, mostly as bicarbonates.
The presence of calcium and magnesium compounds makes the water less likely to produce a lather when soap is used. When it becomes difficult to get a lather, usually when the water contains more than 120 milligrams of calcium and magnesium salts in each liter of water, the water is described as hard. It is common to find hard water
associated with river water and groundwater supplies in areas of limestone or chalk rock.
If the water hardness is mainly from calcium bicarbonate, then the water is called
temporarily hard water. If the water is boiled, the bicarbonate changes to carbonate
and is precipitated out as, for example, the fur on a kettle element. This is a permanent
chemical reaction.
All other compounds of calcium and
magnesium produce permanent hardness because boiling does not bring them out of solution.
Instead, they prevent soap (but not detergents) from producing a lather.
Breaking up water molecules into hydrogen and oxygen
Water will not readily dissociate, or break up, into hydrogen and oxygen gases
unless a large amount of energy is applied, as, for example, when a large
electric current is sent through it. This explains why water does not break up
into gases when heated, but instead changes from liquid to gas.
55