Page 52 - Curriculum Visions Dynamic Book
P. 52
does keep many water molecules from evaporating from the surface. This technique is used in places with hot climates, such as Arizona, where loss of water from reservoirs can be a major problem.
Water reactions that give out heat
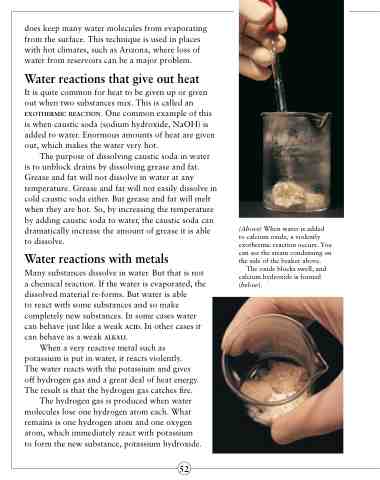
It is quite common for heat to be given up or given out when two substances mix. This is called an exothermic reaction. One common example of this is when caustic soda (sodium hydroxide, NaOH) is added to water. Enormous amounts of heat are given out, which makes the water very hot.
The purpose of dissolving caustic soda in water is to unblock drains by dissolving grease and fat. Grease and fat will not dissolve in water at any temperature. Grease and fat will not easily dissolve in cold caustic soda either. But grease and fat will melt when they are hot. So, by increasing the temperature by adding caustic soda to water, the caustic soda can dramatically increase the amount of grease it is able to dissolve.
Water reactions with metals
Many substances dissolve in water. But that is not a chemical reaction. If the water is evaporated, the dissolved material re-forms. But water is able
to react with some substances and so make completely new substances. In some cases water can behave just like a weak acid. In other cases it can behave as a weak alkali.
When a very reactive metal such as potassium is put in water, it reacts violently.
The water reacts with the potassium and gives off hydrogen gas and a great deal of heat energy. The result is that the hydrogen gas catches fire.
The hydrogen gas is produced when water molecules lose one hydrogen atom each. What remains is one hydrogen atom and one oxygen atom, which immediately react with potassium to form the new substance, potassium hydroxide.
(Above) When water is added to calcium oxide, a violently exothermic reaction occurs. You can see the steam condensing on the side of the beaker above.
The oxide blocks swell, and calcium hydroxide is formed (below).
52