Page 8 - Curriculum Visions Dynamic Book
P. 8
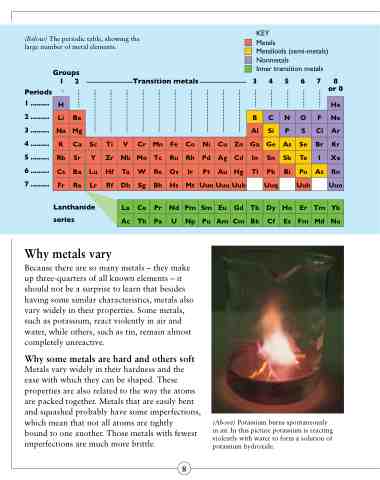
(Below) The periodic table, showing the large number of metal elements.
Groups
KEY Metals
Metalloids (semi-metals) Nonmetals
Inner transition metals
Periods 1 .........
1 2 Transition metals 3 4 5 6 7 8 or 0
H He
2 .........
3 ......... Na Mg
B C N O F Ne Al Si P S Cl Ar
Li Be
4......... K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
5......... Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
6 ......... 7 .........
series
Why metals vary
Because there are so many metals – they make up three-quarters of all known elements – it should not be a surprise to learn that besides having some similar characteristics, metals also vary widely in their properties. Some metals, such as potassium, react violently in air and water, while others, such as tin, remain almost completely unreactive.
Why some metals are hard and others soft
Metals vary widely in their hardness and the ease with which they can be shaped. These properties are also related to the way the atoms are packed together. Metals that are easily bent and squashed probably have some imperfections, which mean that not all atoms are tightly
bound to one another. Those metals with fewest imperfections are much more brittle.
...........
...........
...........
...........
........... ........................... ........................... ........................... ........................... ........................... ........................... ........................... ........................... ........................... ........................... ...........
..
Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Lr Rf Db Sg Bh Hs Mt UunUuuUub Uuq Uuh Uuo
Lanthanide La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No
(Above) Potassium burns spontaneously in air. In this picture potassium is reacting violently with water to form a solution of potassium hydroxide.
8