Page 6 - Curriculum Visions Dynamic Book
P. 6
How metals are made
The properties of a metal are determined by its atomic makeup. When we look at the arrangement of atoms in a metal, we find that they are mostly in the form of crystals. Metals are also usually solid at room temperature.
Pure metals are made of simple crystals. Many are made of sheets of closely packed atoms or electrically charged particles called ions.
(Right) You cannot normally see metal crystals because they are too small. You only notice crystals when they grow under special conditions. For example, barbed wire is often zinc coated (this is called galvanizing), but the zinc crystals do not show. The top of the recycling container below is also zinc coated, but during its manufacture the zinc crystals were given the opportunity to grow. They show as
a speckled pattern on the lid. Look more closely, and you can see the interlocking crystals very clearly.
All atoms have two parts: a central area, or nucleus, made of neutrons and protons, and, moving around the nucleus, a number of electrons. The electrons move in special paths called orbits, known to chemists as shells. Some electrons are found in each shell, and there is a fixed maximum number of electrons that can
fit into any shell. In the case of most metals each of the inner shells has
a full set of electrons, but the outer
shell has only half the electrons that
it could possibly hold.
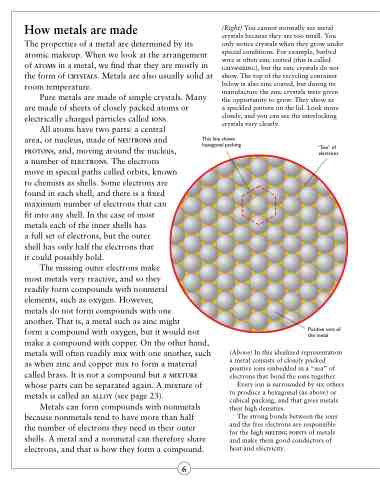
This line shows hexagonal packing
“Sea” of electrons
The missing outer electrons make
most metals very reactive, and so they
readily form compounds with nonmetal
elements, such as oxygen. However,
metals do not form compounds with one
another. That is, a metal such as zinc might
form a compound with oxygen, but it would not make a compound with copper. On the other hand, metals will often readily mix with one another, such as when zinc and copper mix to form a material called brass. It is not a compound but a mixture whose parts can be separated again. A mixture of metals is called an alloy (see page 23).
Metals can form compounds with nonmetals because nonmetals tend to have more than half the number of electrons they need in their outer shells. A metal and a nonmetal can therefore share electrons, and that is how they form a compound.
Positive ions of the metal
(Above) In this idealized representation a metal consists of closely packed positive ions embedded in a “sea” of electrons that bond the ions together.
Every ion is surrounded by six others to produce a hexagonal (as above) or cubical packing, and that gives metals their high densities.
The strong bonds between the ions and the free electrons are responsible for the high melting points of metals and make them good conductors of heat and electricity.
6