Page 44 - Curriculum Visions Dynamic Book
P. 44
Key facts about...
Oxygen
Found in two gaseous forms O2 and O3 (ozone)
Important for burning fuels
Combines with hydrogen to make water
Forms the minerals that make rocks
Part of the tissues of all living organisms
A colourless gas, chemical symbol O
Atomic number 8, atomic weight about 16
Most plentiful element at the surface of the Earth
Essential for breathing
Has no taste
Has no smell
Essential for converting food into energy
Makes up 21% of the atmosphere
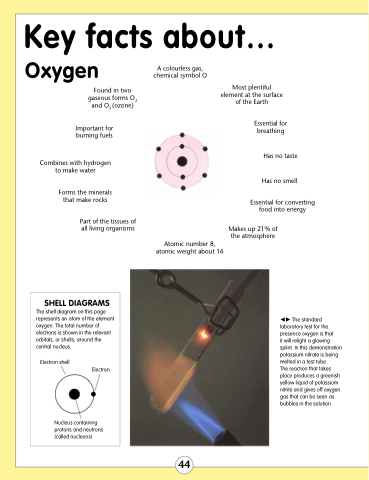
SHELL DIAGRAMS
The shell diagram on this page represents an atom of the element oxygen. The total number of electrons is shown in the relevant orbitals, or shells, around the central nucleus.
Electron shell
Electron
Nucleus containing protons and neutrons (called nucleons)
44
The standard laboratory test for the presence oxygen is that
it will relight a glowing splint. In this demonstration potassium nitrate is being melted in a test tube.
The reaction that takes place produces a greenish yellow liquid of potassium nitrite and gives off oxygen gas that can be seen as bubbles in the solution.