Page 43 - Curriculum Visions Dynamic Book
P. 43
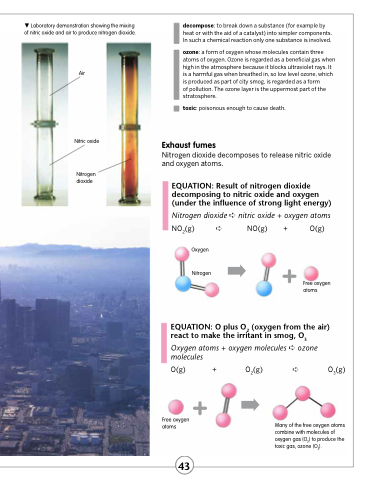
Laboratory demonstration showing the mixing of nitric oxide and air to produce nitrogen dioxide.
Air
Nitric oxide
Nitrogen dioxide
decompose: to break down a substance (for example by heat or with the aid of a catalyst) into simpler components. In such a chemical reaction only one substance is involved.
ozone: a form of oxygen whose molecules contain three atoms of oxygen. Ozone is regarded as a beneficial gas when high in the atmosphere because it blocks ultraviolet rays. It is a harmful gas when breathed in, so low level ozone, which is produced as part of city smog, is regarded as a form
of pollution. The ozone layer is the uppermost part of the stratosphere.
toxic: poisonous enough to cause death.
Exhaust fumes
Nitrogen dioxide decomposes to release nitric oxide and oxygen atoms.
EQUATION: Result of nitrogen dioxide decomposing to nitric oxide and oxygen (under the influence of strong light energy)
Nitrogen dioxide ➪ nitric oxide + oxygen atoms NO2(g) ➪ NO(g) + O(g)
Oxygen
Nitrogen ➡ + Free oxygen atoms
EQUATION: O plus O2 (oxygen from the air) react to make the irritant in smog, O3
Oxygen atoms + oxygen molecules ➪ ozone molecules
O(g) +
Free oxygen + atoms
O2(g) ➪ O3(g)
➡
Many of the free oxygen atoms combine with molecules of oxygen gas (O2) to produce the toxic gas, ozone (O3).
43
43