Page 44 - Curriculum Visions Dynamic Book
P. 44
Key facts about...
Lead
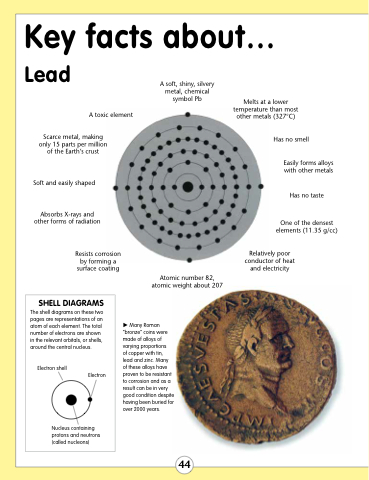
Scarce metal, making only 15 parts per million of the Earth’s crust
Soft and easily shaped
Absorbs X-rays and other forms of radiation
Resists corrosion by forming a surface coating
A soft, shiny, silvery metal, chemical symbol Pb
Melts at a lower temperature than most other metals (327°C)
Has no smell
Easily forms alloys with other metals
Has no taste
One of the densest elements (11.35 g/cc)
Relatively poor conductor of heat and electricity
A toxic element
Atomic number 82, atomic weight about 207
Many Roman “bronze” coins were made of alloys of varying proportions
of copper with tin, lead and zinc. Many of these alloys have proven to be resistant to corrosion and as a result can be in very good condition despite having been buried for over 2000 years.
SHELL DIAGRAMS
The shell diagrams on these two pages are representations of an atom of each element. The total number of electrons are shown in the relevant orbitals, or shells, around the central nucleus.
Electron shell
Electron
Nucleus containing protons and neutrons (called nucleons)
44