Page 16 - Curriculum Visions Dynamic Book
P. 16
Reactivity of lead
Many metals are subject to corrosion when placed in air or soil that is damp. The more reactive elements, such as sodium, cannot be used at all. Others
that are quite reactive, such as iron,
need to be protected by covering them
in a protective coating of, say, paint.
Metal elements that are not very reactive can find many useful applications in weather-beaten environments, or where resistance to corrosion by water is needed.
All metal elements can be placed in order of their reactivity with other elements. The list of the more common metal elements
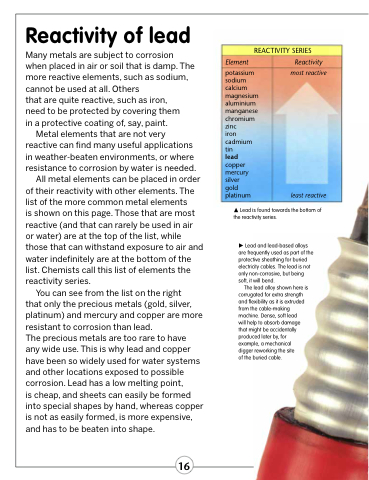
is shown on this page. Those that are most reactive (and that can rarely be used in air or water) are at the top of the list, while those that can withstand exposure to air and water indefinitely are at the bottom of the list. Chemists call this list of elements the reactivity series.
You can see from the list on the right
that only the precious metals (gold, silver, platinum) and mercury and copper are more resistant to corrosion than lead.
The precious metals are too rare to have
any wide use. This is why lead and copper have been so widely used for water systems and other locations exposed to possible corrosion. Lead has a low melting point,
is cheap, and sheets can easily be formed into special shapes by hand, whereas copper is not as easily formed, is more expensive, and has to be beaten into shape.
16 16
REACTIVITY SERIES
Element
potassium sodium calcium magnesium aluminium manganese chromium zinc
iron cadmium tin
lead copper mercury silver gold platinum
Reactivity most reactive
Lead is found towards the bottom of the reactivity series.
Lead and lead-based alloys are frequently used as part of the protective sheathing for buried electricity cables. The lead is not only non-corrosive, but being soft, it will bend.
The lead alloy shown here is corrugated for extra strength and flexibility as it is extruded from the cable-making machine. Dense, soft lead
will help to absorb damage that might be accidentally produced later by, for example, a mechanical digger reworking the site
of the buried cable.
least reactive