Page 10 - Curriculum Visions Dynamic Book
P. 10
Processing lead ore
Most lead is recovered from lead sulphide ore. The first stage of processing involves the concentration of the ore and the removal of sulphur. The ore contains a variety of other elements, each of which has to be removed.
Because of the various impurities, lead
is refined in a number of stages. Each stage depends on the contrast between the chemical and physical properties of lead
and those of the other elements.
Processing lead ore
Lead ore is concentrated by crushing it to a fine powder and placing it into a vat with about three times as much water that contains wetting and frothing chemicals.
Air is introduced into the vat, causing the frothing agents to make bubbles. The chemicals prevent the metal from becoming wet and metal grains are caught up by air bubbles, float, and can be skimmed off.
Flotation increases the concentration of metal to about three-fifths. At this concentration the ore can be roasted – a process called sintering – which removes any sulphur as sulphur dioxide gas, leaving lead oxide behind. The byproduct of the sintering operation, sulphur dioxide gas, is usually converted to sulphuric acid and sold.
Next, the lead oxide is smelted with a supply of coke and limestone in a furnace. The coke is almost pure carbon, and the reaction reduces the lead oxide to lead and forms carbon monoxide gas. The limestone forms a slag with some other impurities, and the metal flows free. It is tapped off the furnace and made into ingots.
The ingots make “base bullion”. This is not pure lead but contains small amounts of other metals. These are separated out and recovered when the lead is purified by the Parkes process (described opposite).
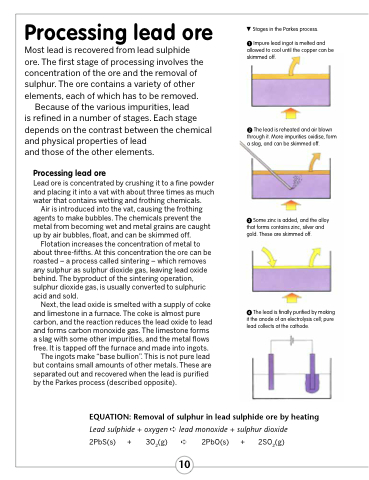
Stages in the Parkes process.
Impure lead ingot is melted and allowed to cool until the copper can be skimmed off.
The lead is reheated and air blown through it. More impurities oxidise, form a slag, and can be skimmed off.
Some zinc is added, and the alloy that forms contains zinc, silver and gold. These are skimmed off.
The lead is finally purified by making it the anode of an electrolysis cell; pure lead collects at the cathode.
EQUATION: Removal of sulphur in lead sulphide ore by heating
Lead sulphide + oxygen ➪ lead monoxide + sulphur dioxide 2PbS(s) + 3O2(g) ➪ 2PbO(s) + 2SO2(g)
10