Page 25 - Curriculum Visions Dynamic Book
P. 25
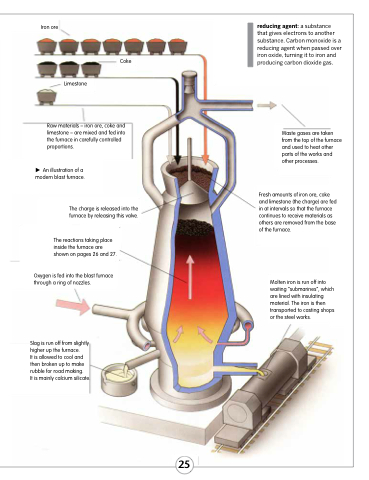
Iron ore
Limestone
Coke
reducing agent: a substance that gives electrons to another substance. Carbon monoxide is a reducing agent when passed over iron oxide, turning it to iron and producing carbon dioxide gas.
Waste gases are taken from the top of the furnace and used to heat other parts of the works and other processes.
Fresh amounts of iron ore, coke and limestone (the charge) are fed in at intervals so that the furnace continues to receive materials as others are removed from the base of the furnace.
Molten iron is run off into waiting “submarines”, which are lined with insulating material. The iron is then transported to casting shops or the steel works.
Raw materials – iron ore, coke and limestone – are mixed and fed into the furnace in carefully controlled proportions.
An illustration of a modern blast furnace.
The charge is released into the furnace by releasing this valve.
The reactions taking place inside the furnace are shown on pages 26 and 27.
Oxygen is fed into the blast furnace through a ring of nozzles.
Slag is run off from slightly higher up the furnace.
It is allowed to cool and then broken up to make rubble for road making.
It is mainly calcium silicate.
25