Page 29 - Curriculum Visions Dynamic Book
P. 29
acidity: a general term for the strength of an acid in a solution.
anion: a negatively charged atom or group of atoms.
cation: a positively charged atom or group of atoms.
ion: an atom, or group of atoms, that has gained or lost one or more electrons and so developed an electrical charge.
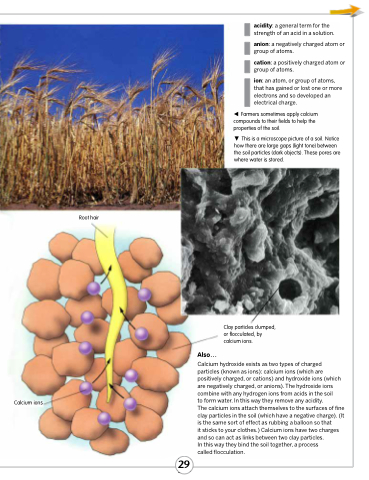
Farmers sometimes apply calcium compounds to their fields to help the properties of the soil.
This is a microscope picture of a soil. Notice how there are large gaps (light tone) between the soil particles (dark objects). These pores are where water is stored.
Calcium ions
Clay particles clumped, or flocculated, by calcium ions.
Also...
Calcium hydroxide exists as two types of charged particles (known as ions): calcium ions (which are positively charged, or cations) and hydroxide ions (which are negatively charged, or anions). The hydroxide ions combine with any hydrogen ions from acids in the soil
to form water. In this way they remove any acidity.
The calcium ions attach themselves to the surfaces of fine clay particles in the soil (which have a negative charge). (It is the same sort of effect as rubbing a balloon so that
it sticks to your clothes.) Calcium ions have two charges and so can act as links between two clay particles.
In this way they bind the soil together, a process
called flocculation.
29 29
Root hair