Page 28 - Curriculum Visions Dynamic Book
P. 28
Calcium in the soil
Calcium compounds are used in a soil to improve
its condition and to balance any acidity. This means keeping the soil materials clumped together into crumb-sized pieces so that the soil drains well and air can get in. For this purpose calcium compounds are used as a form of “glue,” holding the fine clay particles clumped into the size of sand grains.
Several compounds of calcium are used for this purpose. The fastest-acting compound is calcium hydroxide (slaked lime, often just called lime by gardeners), which readily breaks down in the soil.
A more slow-acting compound is crushed limestone, calcium carbonate.
Lime is not always the best choice for a soil conditioner, however, because it will make a
soil alkaline, and plants do not like too alkaline conditions any more than they like acid conditions. This is why it is now more common to apply finely crushed limestone. The limestone will simply remain in the soil until it is dissolved by acid water. In effect the crushed limestone is ready to neutralize the soil when needed but does not cause any damage when not required.
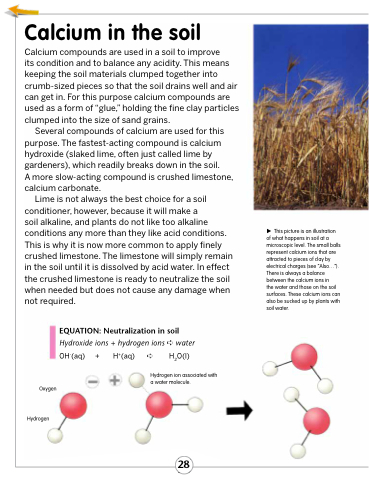
EQUATION: Neutralization in soil
Hydroxide ions + hydrogen ions ➪ water OH-(aq) + H+(aq) ➪ H2O(l)
This picture is an illustration of what happens in soil at a microscopic level. The small balls represent calcium ions that are attracted to pieces of clay by electrical charges (see “Also...”). There is always a balance between the calcium ions in
the water and those on the soil surfaces. These calcium ions can also be sucked up by plants with soil water.
Oxygen
Hydrogen
Hydrogen ion associated with a water molecule.
28 28