Page 6 - Curriculum Visions Dynamic Book
P. 6
Sodium
Sodium is a soft, silvery-coloured metal that can be obtained by passing an electric current through molten table salt (sodium chloride).
Sodium does not occur
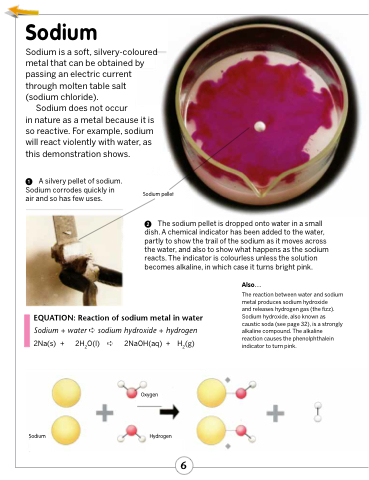
in nature as a metal because it is so reactive. For example, sodium will react violently with water, as this demonstration shows.
A silvery pellet of sodium. Sodium corrodes quickly in air and so has few uses.
Sodium pellet
The sodium pellet is dropped onto water in a small dish. A chemical indicator has been added to the water, partly to show the trail of the sodium as it moves across the water, and also to show what happens as the sodium reacts. The indicator is colourless unless the solution becomes alkaline, in which case it turns bright pink.
EQUATION: Reaction of sodium metal in water
Sodium + water ➪ sodium hydroxide + hydrogen
Also...
The reaction between water and sodium metal produces sodium hydroxide
and releases hydrogen gas (the fizz). Sodium hydroxide, also known as caustic soda (see page 32), is a strongly alkaline compound. The alkaline reaction causes the phenolphthalein indicator to turn pink.
2Na(s) +
2H O(l) 2
➪
2NaOH(aq) +
Oxygen
H (g) 2
Sodium
Hydrogen
6 6