Page 5 - Curriculum Visions Dynamic Book
P. 5
All in all, we consume about a hundred million tonnes of sodium compounds every year.
But although you can see sodium in many compounds, it is rare to see the soft, silvery- coloured metal that is pure sodium because it reacts violently in air and water.
In nature, sodium is always found combined with other elements.
Potassium
Potassium, the twentieth most common element in the Universe and the seventh most common on Earth, is a lightweight, soft, silvery-coloured metal, whose name comes from the word potash. The chemical symbol, K, however, comes from the Latin word kalium, which in turn comes from the Arabic word for alkali.
Potassium and sodium have many similar properties and occur in the
same group in the pattern of elements called the Periodic Table (see page 46). Like sodium, potassium is too reactive
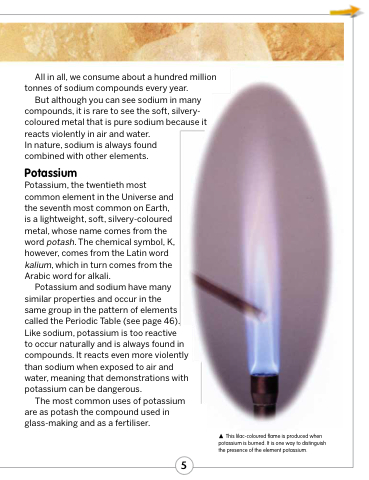
to occur naturally and is always found in compounds. It reacts even more violently than sodium when exposed to air and water, meaning that demonstrations with potassium can be dangerous.
The most common uses of potassium are as potash the compound used in glass-making and as a fertiliser.
5 5
This lilac-coloured flame is produced when potassium is burned. It is one way to distinguish the presence of the element potassium.