Page 32 - Curriculum Visions Dynamic Book
P. 32
Bases
Bases make up a large and important group
of chemicals. If a base is water-soluble it
gives an alkaline reaction when tested with
a chemical indicator. Bases are metal oxides and hydroxides, and nearly all are insoluble. Bases contain hydroxide ions, an ion consisting of one hydrogen and one oxygen atom.
If a base is soluble, like sodium hydroxide (caustic soda), it is called an alkali. An alkali
has an excess of hydroxide ions (the opposite
to an acid, which has an excess of hydrogen ions). Like an acid, a strong alkali will completely dissociate in water, releasing the ions of its metal and its hydroxide.
In general, a base will react with an acid to form a new substance (called a salt) and water but no additional compounds. Sodium chloride (common salt) is a neutral substance that will show neither acid nor alkaline response when tested with a chemical indicator.
Examples of bases are ammonia (NH3, a
gas in solution in water used as a cleaning agent and in fertilisers), lime (CaO, a solid
used in cement and fertilisers), magnesium hydroxide (Mg(OH)2, a solid used as an antacid for indigestion) and sodium hydroxide (NaOH,
a solid used in oven cleaners and soap-making). Some bases, such as sodium hydroxide, are caustic and harmful to the skin.
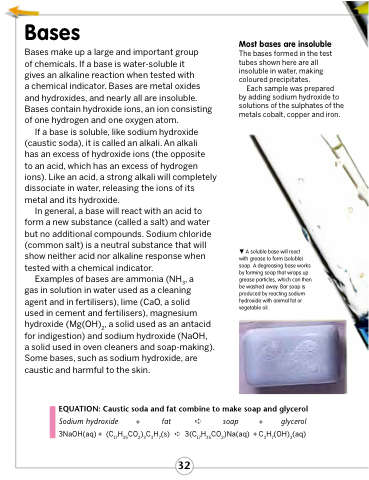
Most bases are insoluble
The bases formed in the test tubes shown here are all insoluble in water, making coloured precipitates.
Each sample was prepared by adding sodium hydroxide to solutions of the sulphates of the metals cobalt, copper and iron.
A soluble base will react
with grease to form (soluble) soap. A degreasing base works by forming soap that wraps up grease particles, which can then be washed away. Bar soap is produced by reacting sodium hydroxide with animal fat or vegetable oil.
EQUATION: Caustic soda and fat combine to make soap and glycerol
Sodium hydroxide + fat ➪ soap + glycerol 3NaOH(aq)+ (C17H35CO2)3C3H7(s) ➪ 3(C17H35CO2)Na(aq) +C3H7(OH)3(aq)
32