Page 23 - Curriculum Visions Dynamic Book
P. 23
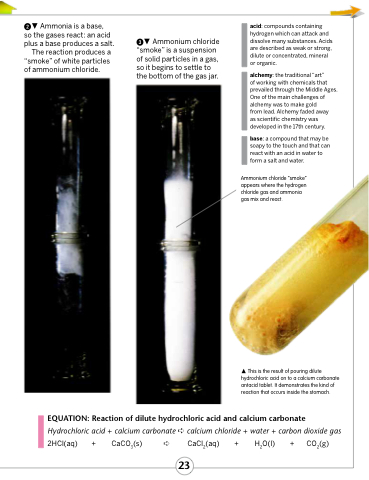
Ammonia is a base,
so the gases react: an acid plus a base produces a salt.
The reaction produces a “smoke” of white particles of ammonium chloride.
Ammonium chloride “smoke” is a suspension of solid particles in a gas, so it begins to settle to the bottom of the gas jar.
acid: compounds containing hydrogen which can attack and dissolve many substances. Acids are described as weak or strong, dilute or concentrated, mineral or organic.
alchemy: the traditional “art”
of working with chemicals that prevailed through the Middle Ages. One of the main challenges of alchemy was to make gold
from lead. Alchemy faded away
as scientific chemistry was developed in the 17th century.
base: a compound that may be soapy to the touch and that can react with an acid in water to form a salt and water.
Ammonium chloride “smoke” appears where the hydrogen chloride gas and ammonia gas mix and react.
EQUATION: Reaction of dilute hydrochloric acid and calcium carbonate
Hydrochloric acid + calcium carbonate ➪ calcium chloride + water + carbon dioxide gas 2HCl(aq) + CaCO3(s) ➪ CaCl2(aq) + H2O(l) + CO2(g)
This is the result of pouring dilute hydrochloric acid on to a calcium carbonate antacid tablet. It demonstrates the kind of reaction that occurs inside the stomach.
23