Page 17 - Curriculum Visions Dynamic Book
P. 17
Limitations of the Haber–Bosch process
Although the Haber–Bosch process has produced the foundation material for important fertilisers and has thus greatly contributed to the increase in the world’s food supply, it consumes large amounts of energy and the equipment, operating at high temperatures and pressures, is expensive to manufacture.
The source of hydrogen for this reaction is mainly petroleum, a nonrenewable fossil fuel. For these reasons, fertilisers are expensive and scientists are looking for a natural way to fix nitrogen for use as a fertiliser, especially for use in the developing world.
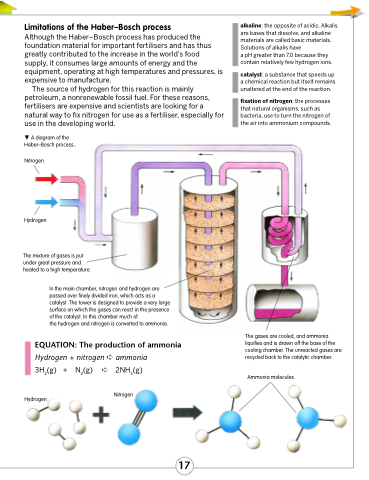
A diagram of the Haber–Bosch process.
Nitrogen
Hydrogen
The mixture of gases is put under great pressure and heated to a high temperature.
In the main chamber, nitrogen and hydrogen are passed over finely divided iron, which acts as a catalyst. The tower is designed to provide a very large surface on which the gases can react in the presence of the catalyst. In this chamber much of
the hydrogen and nitrogen is converted to ammonia.
EQUATION: The production of ammonia
Hydrogen + nitrogen ➪ ammonia 3H2(g) + N2(g) ➪ 2NH3(g)
alkaline: the opposite of acidic. Alkalis are bases that dissolve, and alkaline materials are called basic materials. Solutions of alkalis have
a pH greater than 7.0 because they contain relatively few hydrogen ions.
catalyst: a substance that speeds up a chemical reaction but itself remains unaltered at the end of the reaction.
fixation of nitrogen: the processes that natural organisms, such as bacteria, use to turn the nitrogen of the air into ammonium compounds.
The gases are cooled, and ammonia liquifies and is drawn off the base of the cooling chamber. The unreacted gases are recycled back to the catalytic chamber.
Ammonia molecules
Hydrogen
Nitrogen
17